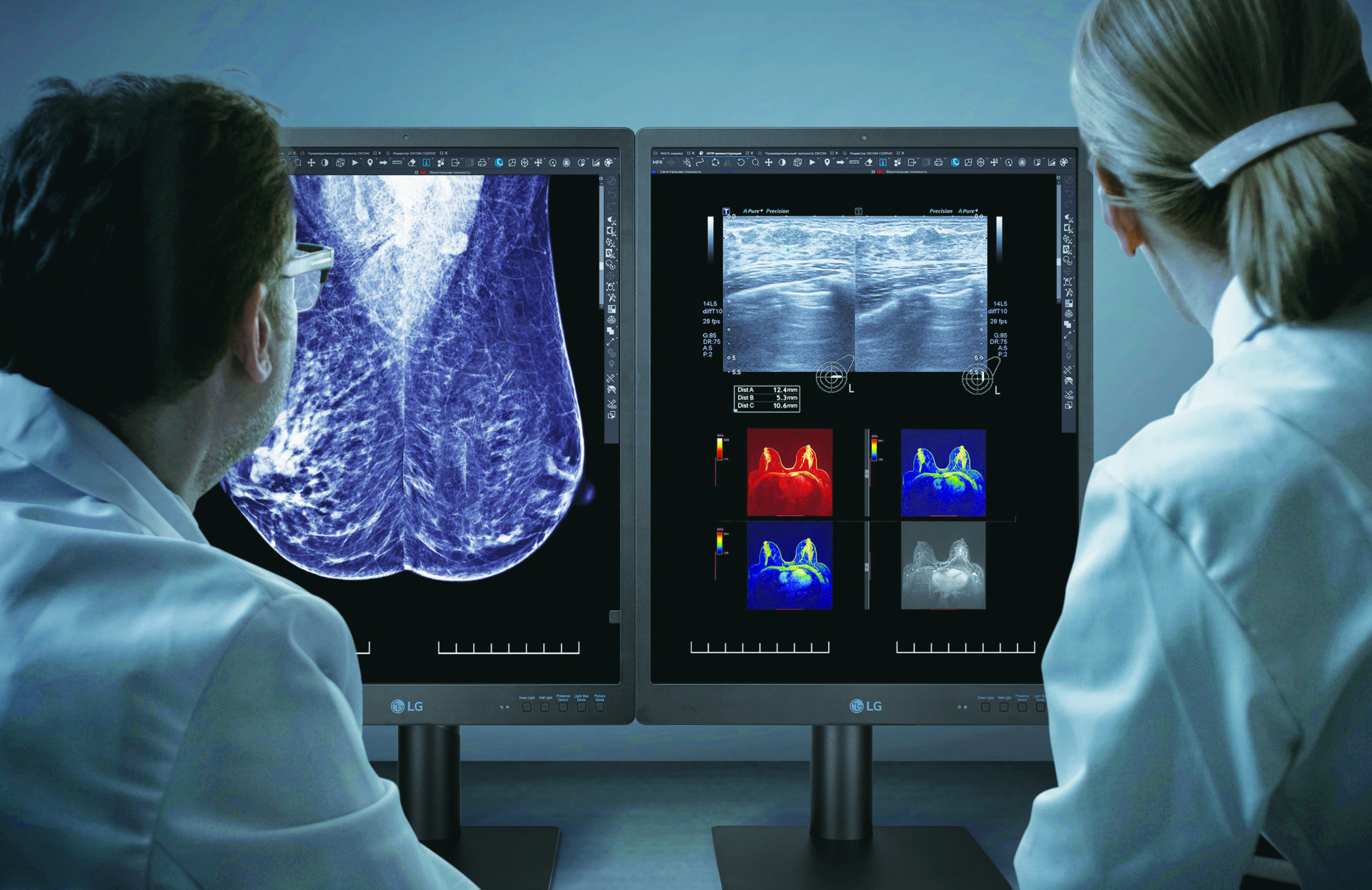
Healthcare businesses in the U.S will soon have a new FDA-approved electronic device option with the introduction of the LG 21HQ613D.
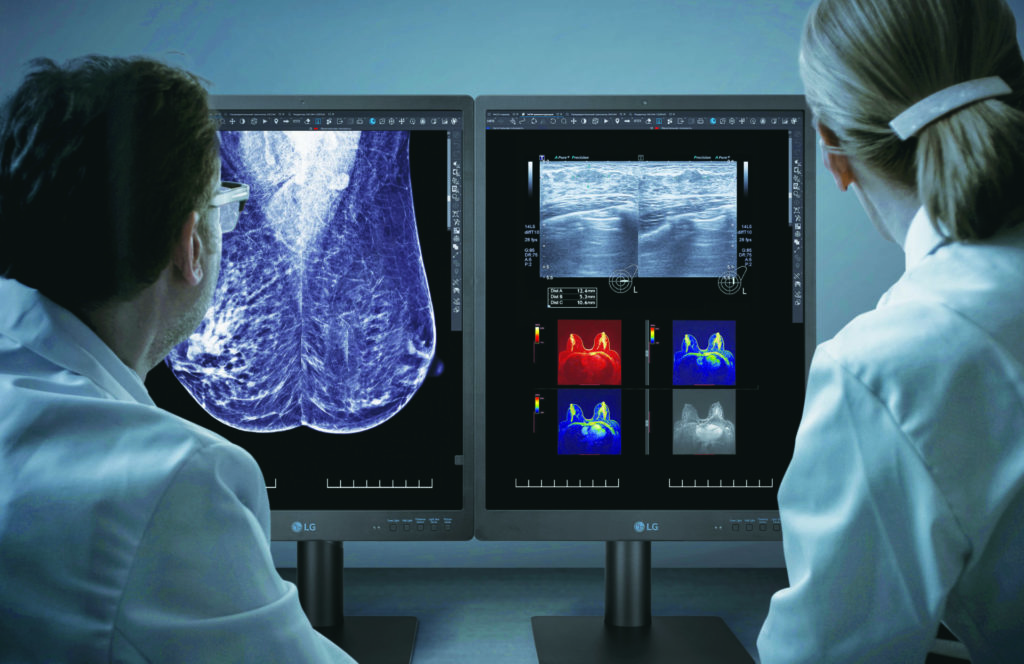 Tailored specifically for mammography and breast tomosynthesis, the 21HQ613D boasts a 21.3-inch form factor housing an IPS panel capable of delivering 1100cd/m² of luminance, enhancing color contrast and background distinction.
Tailored specifically for mammography and breast tomosynthesis, the 21HQ613D boasts a 21.3-inch form factor housing an IPS panel capable of delivering 1100cd/m² of luminance, enhancing color contrast and background distinction.
With its unconventional 2048×2560 resolution, this portrait-style monitor is optimized for MRIs, CT scans, and ultrasounds. An integrated front-side sensor eliminates the need for external calibration by automatically adjusting settings.
For further calibration and quality assurance, the LG Calibration Studio Medical software offers DICOM calibration, ensuring compliance with international QC standards for medical monitors.
To make fellow researchers and practitioners’ lives easier, the 21HQ613D features Focus View Mode and Pathology Mode, catering to a wide array of applications. Focus View Mode enables zooming in and out on specific areas while dimming surrounding regions for improved focus, while Pathology Mode prioritizes clarity and precision. Users can also adjust between 2MP, 3MP, and 5MP resolutions to match the displayed image.
Additional features include daisy-chainable DisplayPort connectivity and flexible tilt, swivel, height, and pivot adjustments for optimal setup versatility. Five customizable hotkeys provide quick access to various settings.
For environmentally-conscious organizations, the monitor automatically dims after 5 minutes of inactivity, while Down Light and Wall Light features offer additional illumination for reading in low-light conditions. Alternatively, the Auto Luminance sensor automates brightness adjustment for hassle-free operation.